Our eyes are a key sense organ that sends information about our surroundings to the brain. Light passes through the transparent cornea and is focused by the lens, after which it is converted into electrical signals by photoreceptors in the retina. These signals are then sent to the brain for image processing through the optic nerve comprising the axons of retinal ganglion cells (RGCs). In humans, there are various degenerative eye diseases that are still lacking effective treatments. These include Fuchs cornea endothelial dystrophy (FCED), cataracts, glaucoma, retinitis pigmentosa (RP), and age-related macular degeneration (AMD). The ciliary body (CB) lies at the periphery of the retina and it secretes aqueous humour, which maintains a normal intraocular pressure (IOP). It is known that an elevated IOP is an important risk factor for glaucoma. Recently, the Xie laboratory demonstrated that the CB produces an ocular “niche” through secretion for promoting the development and maintenance of the cornea, lens, and retina. This suggests that a defective CB might contribute to the pathogenesis of various degenerative eye diseases. The Xie team uses mouse genetic models to study the mechanisms underlying degenerative eye diseases and develop treatments. Here are their current research accomplishments and their future plans for studying eye development and diseases:
1. Studying eye development and diseases
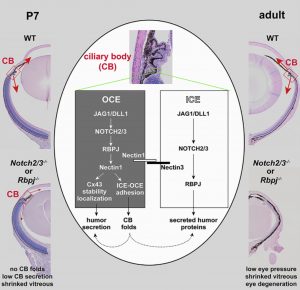
The Xie laboratory uses conditional knockout mice to investigate eye development and diseases. First, they demonstrated that FGF signaling is required for the proliferation of retinal progenitors, and the fate switch and shape changes of progenitor cells in the periphery of the retinal fissure (Cell Research, 2013; IVOS, 2018). They showed that conditionally inactivating FGF signaling in the mouse retina leads to a coloboma-like phenotype. Coloboma is a rare human eye birth defect characterised by severe vision loss. Therefore, the FGF signaling-defective mice are a good model for investigating coloboma in humans in further detail. Second, the Xie team demonstrated that Notch signaling not only promotes CB development, but it also controls the secretion of aqueous humor and vitreous proteins, which are essential for the development and maintenance of the cornea, lens, and retina (PNAS, 2013; Cell Reports, 2021). These findings suggest that defective CB secretion might contribute to various degenerative eye diseases, which opens potential new research directions for uncovering the causes of these diseases. Currently, the Xie laboratory is investigating the causes of glaucoma (caused by a loss of RGCs) and FCED by identifying critical CB-secreted factors. In addition, since defects in the trabecular meshwork (TM) result in a high IOP, a known risk factor for glaucoma, the Xie laboratory is also investigating how TM development is regulated at the molecular and cellular levels.
Research directions:
- To identify CB-secreted proteins and study their functions in the regulation of eye tissue development and maintenance.
- To investigate how TM development is regulated at the molecular and cellular level.
- To identify the molecular mechanisms underlying FCED and glaucoma.
2. Developing stem cell- and gene-based therapies for degenerative eye diseases
Stem cells have been advocated to treat various degenerative diseases. The Xie laboratory showed that in vitro expanded retinal stem cells (RSCs) can generate functional photoreceptors capable of restoring a light response in blind rd1 mutant mice that lack photoreceptors (Cell Research, 2013). However, these transplanted photoreceptors die within 3 months. This suggests that in this case, the persistent disease condition might be a major roadblock to stem cell therapy. Although the RGCs survive and become integrated into the retina, RSC-derived RGCs fail to extend axons to the brain. These obstacles must be overcome before successful stem cell therapies for degenerative eye diseases can be developed. Therefore, halting or slowing the progression of retinal degenerative diseases is still the best strategy for treatment at this time.
We aim to develop AAV-based gene therapies based on the molecular mechanisms underlying the degenerative eye diseases, to prevent their cause and thus avoid their progression into complete vision loss. Following the eradication of the cause, cell transplantation can be applied to restore vision loss in severe cases.
Research directions:
- To use AAV-mediated gene therapy to prevent the loss of RGCs in mouse glaucoma models.
- To use AAV-mediated therapy to prevent the loss of corneal endothelial cells in mouse FCED models.
- To develop combined gene- and stem cell-based therapies for treating FCED and glaucoma models.